The effect of water on the binding of glycosaminoglycan saccharides to hydroxyapatite surfaces: a molecular dynamics study†
Abstract
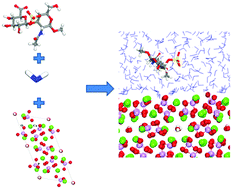
Classical molecular dynamics (MD) simulations have been employed to study the interaction of the saccharides glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc) with the (0001) and (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) surfaces of the mineral hydroxyapatite (HAP). GlcA and GalNAc are the two constituent monosaccharides of the glycosaminoglycan chondroitin sulfate, which is commonly found in bone and cartilage and has been implicated in the modulation of the hydroxyapatite biomineralization process. MD simulations of the mineral surfaces and the saccharides in the presence of solvent water allowed the calculation of the adsorption energies of the saccharides on the HAP surfaces. The calculations show that GalNAc interacts with HAP principally through the sulfate and the carbonyl of acetyl amine groups, whereas the GlcA interacts primarily through the carboxylate functional groups. The mode and strength of the interaction depends on the orientation of the saccharide with respect to the surface and the level of disruption of the layer of water competing with the saccharide for adsorption sites on the HAP surface, suggesting that chondroitin 4-sulfate binds to the layer of solvent water rather than to HAP.
0) surfaces of the mineral hydroxyapatite (HAP). GlcA and GalNAc are the two constituent monosaccharides of the glycosaminoglycan chondroitin sulfate, which is commonly found in bone and cartilage and has been implicated in the modulation of the hydroxyapatite biomineralization process. MD simulations of the mineral surfaces and the saccharides in the presence of solvent water allowed the calculation of the adsorption energies of the saccharides on the HAP surfaces. The calculations show that GalNAc interacts with HAP principally through the sulfate and the carbonyl of acetyl amine groups, whereas the GlcA interacts primarily through the carboxylate functional groups. The mode and strength of the interaction depends on the orientation of the saccharide with respect to the surface and the level of disruption of the layer of water competing with the saccharide for adsorption sites on the HAP surface, suggesting that chondroitin 4-sulfate binds to the layer of solvent water rather than to HAP.

 Please wait while we load your content...
Please wait while we load your content...