An in situ high-temperature scanning electron microscopy study of acanthite–argentite phase transformation in nanocrystalline silver sulfide powder†
Abstract
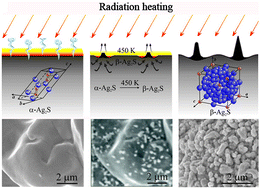
For the first time, the α-Ag2S (acanthite)–β-Ag2S (argentite) phase transformation in nanocrystalline and coarse-crystalline powders of silver sulfide has been observed in situ by the scanning electron microscopy method in real-time. The argentite crystals are formed on the surface of acanthite particles as a result of electron-beam heating. According to the differential thermal analysis data, the transformation occurs at a temperature of ∼449–450 K, and the enthalpy of transformation is equal to ∼3.7–3.9 kJ mol−1. The presence of α-Ag2S (acanthite)–β-Ag2S (argentite) phase transformation is confirmed in situ by high-temperature X-ray diffraction data.

 Please wait while we load your content...
Please wait while we load your content...