Surface oxygen-vacancy induced photocatalytic activity of La(OH)3 nanorods prepared by a fast and scalable method
Abstract
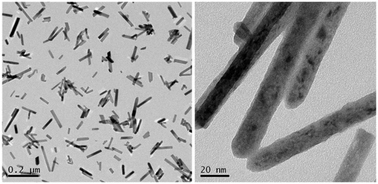
Uniform one-dimensional defective La(OH)3 nanorods were synthesized by a facile, fast and scalable method. This simple method avoids treatment at high temperature, utility of surfactants or templates, and can be finished within a short time. The results indicate that oxygen-vacancies were formed in La(OH)3 nanorods, which could extend the photoresponse range. The XPS, PL, solid state ESR measurements and DFT calculations revealed the pivotal role of oxygen-vacancy in the formation of an impurity level in the band gap of La(OH)3. The as-prepared La(OH)3 nanorods exhibited efficient photocatalytic activity in the removal of NO at the ppb-level under ultraviolet illumination. The highly enhanced photocatalytic activity of La(OH)3 nanorods could be ascribed to the synergy of the lower impurity level below the conduction band and the high separation efficiency of photogenerated electron–hole pairs. DMPO-ESR spin trapping results imply that the hydroxyl radicals are the main reactive species that are responsible for NO photooxidation. On the basis of combined experimental and theoretical investigation, an oxygen vacancy-mediated photocatalysis mechanism of defective La(OH)3 nanorods was proposed. This work could not only provide a fast and environmentally friendly approach for the synthesis of nanostructured photocatalysts, but also new insights into the understanding of the role of vacancy in semiconductor photocatalysis.

 Please wait while we load your content...
Please wait while we load your content...