Adsorption of choline benzoate ionic liquid on graphene, silicene, germanene and boron-nitride nanosheets: a DFT perspective
Abstract
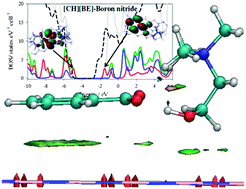
The adsorption of choline benzoate ([CH][BE]) ionic liquid (IL) on the surface of different hexagonal nanosheets has been studied using Density Functional Theory (DFT) methods. For this, the interaction mechanism, binding energies and electronic structure of [CH][BE] ionic liquid on four types of nanosheets, i.e., graphene, silicene, germanene and boron-nitride, were estimated and compared. The adsorption of [CH][BE] ionic liquid on different nanosheets is mainly featured by van der Waals forces, leading to strong benzoate ion–surface π-stacking. Likewise, there is also an important charge transfer from the anion to the sheet. The electronic structure analysis shows that Si- and Ge-based sheets lead to the largest changes in the HOMO and LUMO levels of choline benzoate. This paper provides new insights into the capability of DFT methods to provide useful information about the adsorption of ionic liquids on nanosheets and how ionic liquid features could be tuned through the adsorption on the suitable nanosheet.

 Please wait while we load your content...
Please wait while we load your content...