Molecular mechanism of water permeation in a helium impermeable graphene and graphene oxide membrane†
Abstract
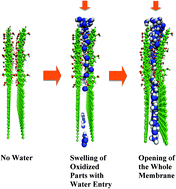
Layers of graphene oxide (GO) are found to be good for the permeation of water but not for helium (Science, 2012, 335(6067), 442–444) suggesting that the GO layers are dynamic in the formation of a permeation route depending on the environment they are in (i.e., water or helium). To probe the microscopic origin of this observation we calculate the potential of mean force (PMF) of GO sheets (with oxidized and reduced parts), with the inter-planar distance as a reaction coordinate in helium and water. Our PMF calculation shows that the equilibrium interlayer distance between the oxidized part of the GO sheets in helium is at 4.8 Å leaving no space for helium permeation. In contrast, the PMF of the oxidized part of the GO in water shows two minima, one at 4.8 Å and another at 6.8 Å, corresponding to no water and a water filled region, thus giving rise to a permeation path. The increased electrostatic interaction between water with the oxidized part of the sheet helps the sheet open up and pushes water inside. Based on the entropy calculations for water trapped between graphene sheets and oxidized graphene sheets at different inter-sheet spacings, we also show the thermodynamics of filling.

 Please wait while we load your content...
Please wait while we load your content...