Microhydrated dihydrogen phosphate clusters probed by gas phase vibrational spectroscopy and first principles calculations†
Abstract
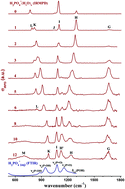
We report infrared multiple photon dissociation (IRMPD) spectra of cryogenically-cooled H2PO4−(H2O)n anions (n = 2–12) in the spectral range of the stretching and bending modes of the solute anion (600–1800 cm−1). The spectra cannot be fully understood using the standard technique of comparison to harmonic spectra of minimum-energy structures; a satisfactory assignment requires considering anharmonic effects as well as entropy-driven hydrogen bond network fluctuations. Aided by finite temperature ab initio molecular dynamics simulations, the observed changes in the position, width and intensity of the IRMPD bands with cluster size are related to the sequence of microsolvation. Due to stronger hydrogen bonding to the two terminal P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, these are hydrated before the two P–OH groups. By n = 6, all four end groups are involved in the hydrogen bond network and by n = 12, the cluster spectra show similarities to the condensed phase spectrum of H2PO4−(aq). Our results reveal some of the microscopic details concerning the formation of the aqueous solvation environment around H2PO4−, provide ample testing grounds for the design of model solvation potentials for this biologically relevant anion, and support a new paradigm for the interpretation of IRMPD spectra of microhydrated ions.
O groups, these are hydrated before the two P–OH groups. By n = 6, all four end groups are involved in the hydrogen bond network and by n = 12, the cluster spectra show similarities to the condensed phase spectrum of H2PO4−(aq). Our results reveal some of the microscopic details concerning the formation of the aqueous solvation environment around H2PO4−, provide ample testing grounds for the design of model solvation potentials for this biologically relevant anion, and support a new paradigm for the interpretation of IRMPD spectra of microhydrated ions.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...