The effect of the size and shape on the bond number of quantum dots and its relationship with thermodynamic properties
Abstract
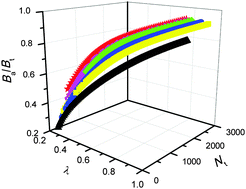
Through introducing the size (Nt) and the shape factor (λ), the size- and shape-dependent bond number Ba of quantum dots, respectively, with icosahedral, truc-decohedral, cuboctahedral, octahedral, decohedral and tetrahedral structures is established in this work. It is found that Nt and λ have reverse contribution to Ba, that is, Ba increases with increase in Nt, while it decreases with increase in λ. As the basic parameter, the size- and shape-dependent Ba function is extended to predict the cohesive energy Ec(Nt) of quantum dots. Similar to Ba, Ec(Nt) shows strong dependence on both the size and shape. Larger Nt leads to higher Ec(Nt), whereas larger λ results in a smaller Ec(Nt) value. There is a sequence: Ec(IH) > Ec(CO) > Ec(truc-DH) > Ec(OT) > Ec(DH) > Ec(TH) if Nt is certain, which is similar to Ba since Ba(IH) > Ba(CO) > Ba(truc-DH) > Ba(OT) > Ba(DH) > Ba(TH) is tested in the whole size range. To some extent, this is due to λ(IH) = λ(truc-DH) < λ(CO) < λ(OT) < λ(DH) < λ(TH), however, Ba(IH) > Ba(truc-DH) despite λ(IH) = λ(truc-DH). In addition, λ is no longer constant and increases with increase in Nt when the shape is given. The fact that whatever the shape is, Ba or Ec(Nt) increases upon increasing Nt, meaning that the shape is a secondary factor if compared with the size. The validity of the size- and shape-related model for the Ec(Nt) function is also confirmed by the simulation results of the size- and shape-dependent thermodynamic stability of Au, Ag, Cu, Ca, Sr, and Si quantum dots with different atomic structures.

 Please wait while we load your content...
Please wait while we load your content...