Oxygen reduction reaction activity and structural stability of Pt–Au nanoparticles prepared by arc-plasma deposition
Abstract
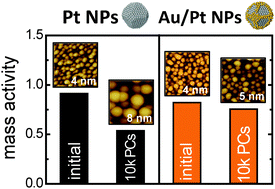
The oxygen reduction reaction (ORR) activity and durability of various Aux/Pt100 nanoparticles (where x is the atomic ratio of Au against Pt) are evaluated herein. The samples were fabricated on a highly-oriented pyrolytic graphite substrate at 773 K through sequential arc-plasma depositions of Pt and Au. The electrochemical hydrogen adsorption charges (electrochemical surface area), particularly the characteristic currents caused by the corner and edge sites of the Pt nanoparticles, decrease with increasing Au atomic ratio (x). In contrast, the specific ORR activities of the Aux/Pt100 samples were dependent on the atomic ratios of Pt and Au: the Au28/Pt100 sample showed the highest specific activity among all the investigated samples (x = 0–42). As for ORR durability evaluated by applying potential cycles between 0.6 and 1.0 V in oxygen-saturated 0.1 M HClO4, Au28/Pt100 was the most durable sample against the electrochemical potential cycles. The results clearly showed that the Au atoms located at coordinatively-unsaturated sites, e.g. at the corners or edges of the Pt nanoparticles, can improve the ORR durability by suppressing unsaturated-site-induced degradation of the Pt nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...