The structure of chloromethyl thiocyanate, CH2ClSCN, in gas and crystalline phases†
Abstract
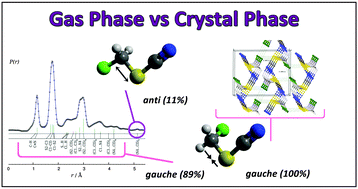
The structural and conformational properties of chloromethyl thiocyanate, CH2ClSCN, were studied in the solid phase and in the gas phase using in situ low-temperature single-crystal X-ray diffraction experiments (XRD) and gas electron diffraction (GED), respectively. Depending on the mutual orientation of the Cl–C bond and the –SCN group, two conformations, gauche and anti, were found to coexist in the gas phase. The gauche conformer, with a dihedral angle φ(ClC–SC) = 71.8(4)°, is the most stable form, with an abundance of 89(3)% at ambient temperature. High level quantum-chemical calculations at the CCSD(T)/cc-pVTZ level of approximation reproduce these experimental results. In the solid state only gauche conformers were found to be present. The crystal structure shows specific intermolecular interactions including chalcogen-type interactions. The experimental electron density distribution was determined by high-angle X-ray diffraction. The atoms in molecules (AIM) theory was applied to analyze the charge density topology for a better characterization of intermolecular interactions present in the crystal.

 Please wait while we load your content...
Please wait while we load your content...