Unique properties of silver cations in solid-acid catalysis by zeolites and heteropolyacids
Abstract
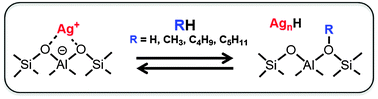
Ag+-exchanged zeolites exhibit unique catalytic properties caused by the combination of their redox and acidic properties. Partial reduction of Ag+ ions in zeolites with hydrogen leads to the formation of acidic protons and silver metal particles, which can be observed using X-ray powder diffraction patterns (XRD). By simply evacuating hydrogen from the system, the silver metal particles are returned back to Ag+ ions and at the same time, acidic protons are eliminated. This interconversion of Ag+ ions and silver metal or gaseous hydrogen and surface protons is reflexed in the catalytic activities of Ag+-exchanged zeolites for acid-catalyzed reactions: the activity of Ag+-exchanged Y zeolite (Ag-Y) reversibly changes with the partial pressure of hydrogen. Furthermore, the activity of Ag-Y in the presence of hydrogen is higher than that of H+-exchanged Y zeolite (H-Y). Similar phenomena are also observed for the silver salt of dodecatungstophosphoric acid (Ag3PW12O40). Ag+-exchanged ZSM-5 zeolite (Ag-ZSM-5) is a very selective catalyst for aromatization of alkanes, alkenes and methanol. Examination of the activation step of lower alkanes revealed that Ag+ ions dramatically enhance the dehydrogenation of the alkanes via heterolytic dissociation of the alkanes into carbenium ions and hydride species. Ag+-exchanged zeolites can also activate methane. The reaction of methane with ethene and benzene gives propene and toluene, respectively. Ag-ZSM-5 is a very stable catalyst under hydrothermal conditions because of the interconversion properties of Ag+ ions and silver metal in the zeolite.

 Please wait while we load your content...
Please wait while we load your content...