Co-electrodeposition of RuO2–MnO2 nanowires and the contribution of RuO2 to the capacitance increase†
Abstract
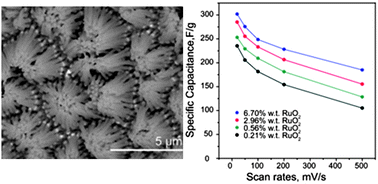
A wide range of metal oxides have been studied as pseudocapitors, with the goal of achieving higher power than traditional batteries and higher energy than traditional capacitors. However, most metal oxides have relatively low conductivity, and the few exceptions, like RuO2, are prohibitively expensive. Mixed metal oxides provided an opportunity to incorporate small amounts of expensive materials to enhance the performance of a less expensive, poorer performing material. Here, by homogeneously co-depositing a small amount of energy dense and conductive RuO2 into MnO2 nanowires, we demonstrate an improvement in specific capacitance. Importantly, we also demonstrate that this improvement is not primarily provided by redox activity of RuO2, but rather by improvement of the composite conductivity. A series of RuO2–MnO2 composite nanowires with different RuO2 loading percentages have been synthesized by performing co-electrodeposition in a porous alumina template. The structure of these RuO2–MnO2 nanowires is characterized by TEM and SEM. EDS mapping shows that RuO2 is well distributed in MnO2 matrix nanowires. The chemical constituents and the phase of these composite nanowires are confirmed by X-ray photoelectron and Raman spectroscopy. The amount of RuO2 is controlled by varying the concentrations of RuCl3 and MnAc2 in the deposition solution. The precise masses of MnO2 and RuO2 are determined by ICP-AES elemental analysis. MnO2 nanowires with 6.70 wt% RuO2 demonstrate a specific capacitance of 302 F g−1 at 20 mV s−1, compared to 210 F g−1 for pristine MnO2 nanowires. Investigation of the RuO2 loading amount effect was conducted by electrochemical impedance spectroscopy (EIS) and deconvolution of capacitances, using methods previously reported by both Dunn and Transsiti. The RuO2–MnO2 nanowires studied here demonstrate a simple, straighforward method to overcome the intrinsically poor conductivity of MnO2, and clarify the source of RuO2's contribution to the improved performance.

 Please wait while we load your content...
Please wait while we load your content...