An important impact of the molecule–electrode coupling asymmetry on the efficiency of bias-driven redox processes in molecular junctions
Abstract
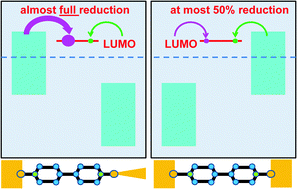
Two recent experimental and theoretical studies (Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1282–1287; Phys. Chem. Chem. Phys., 2014, 16, 25942–25949) have addressed the problem of tuning the molecular charge and vibrational properties of single molecules embedded in nanojunctions. These are molecular characteristics escaping so far from an efficient experimental control in broad ranges. Here, we present a general argument demonstrating why, out of various experimental platforms possible, those wherein active molecules are asymmetrically coupled to electrodes are to be preferred to those symmetrically coupled for achieving a(n almost) complete redox process, and why an electrochemical environment has advantages over “dry” setups. This study aims at helping to nanofabricate molecular junctions using the most appropriate platforms allowing the broadest possible bias-driven control over the redox state and vibrational modes of single molecules linked to electrodes.

 Please wait while we load your content...
Please wait while we load your content...