A new approach to elucidating repair reactions of resveratrol†
Abstract
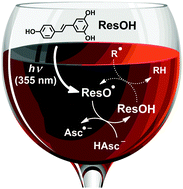
The repair by co-antioxidants of the phenoxy radical of resveratrol, the famous health-preserving ingredient of red wine, is a key step of radical scavenging cascades in nature. To generate that radical, we employed 355 nm photoionization as a direct and selective access that reduces the chemical complexity and is equally applicable in organized phases; to monitor it, we used its hitherto unreported absorption in the red where no other species in our systems interfere. With this novel approach, we measured rate constants and H/D kinetic isotope effects for the repairs by ascorbate, trolox (a vitamin E analogue) and 4-aminophenol, and identified the mechanisms as one-step hydrogen abstractions. Cysteine and glutathione are unreactive. In micellar solution (SDS), the repair by ascorbate is much slower and involves only the hydrophilic phenoxy moieties protruding from the micelles. The new experimental strategy also led to a reevaluation of extinction coefficients, rate constants and mechanisms.

 Please wait while we load your content...
Please wait while we load your content...