Intermolecular carbon–carbon, nitrogen–nitrogen and oxygen–oxygen non-covalent bonding in dipolar molecules†‡
Abstract
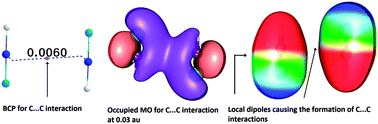
Clear evidence for the existence of intermolecular carbon–carbon (C⋯C), nitrogen–nitrogen (N⋯N) and oxygen–oxygen (O⋯O) interactions between atoms in similar chemical environments in homogeneous dimers of organic dipolar molecules has been obtained from molecular orbital (MO), natural bond orbital (NBO) and atoms-in-molecule (AIM) electron density analyses at the M06L/6-311++G(d,p) level of density functional theory (DFT). These X⋯X type interactions are mainly the result of local polarization effects, causing segregation of electron-rich and electron-deficient regions in the X atoms, leading to complementary electrostatic interactions. NBO analysis provides evidence of charge transfer between the two X atoms. Even in symmetrical molecules such as acetylene, induced dipoles in the dimer create C⋯C bonding interactions. The strength of this type of interaction increases with increase in the dipole moment of the molecule. Energy decomposition analysis (EDA) shows that the electrostatic component of the interaction energy (Eint) is very high, up to 95.86%. The C⋯C interactions between similar carbon atoms are located for several crystal structures obtained from the literature. In addition, MO, AIM and electrostatic potential analyses support interactions between similar oxygen (O⋯O) and nitrogen (N⋯N) atoms in a variety of molecular dimers. Good prediction of Eint is achieved in terms of the total gain in electron density at non-covalently interacting intermolecular bonds (∑ρ) and the monomer dipole moment (μ). A rigorously tested QSAR equation has been derived to predict Eint for all dimer systems:

 Please wait while we load your content...
Please wait while we load your content...