Structural characterization of gas-phase cysteine and cysteine methyl ester complexes with zinc and cadmium dications by infrared multiple photon dissociation spectroscopy†
Abstract
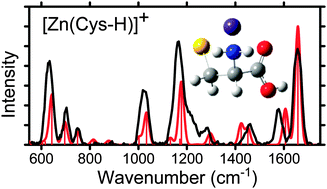
Structural characterization of gas-phase ions of cysteine (Cys) and cysteine methyl ester (CysOMe) complexed to zinc and cadmium is investigated by infrared multiple photon dissociation (IRMPD) action spectroscopy using a free electron laser in combination with density functional theory calculations. IRMPD spectra are measured for [Zn(Cys-H)]+, [Cd(Cys-H)]+, [Zn(CysOMe-H)]+, [Cd(CysOMe-H)]+ and CdCl+(CysOMe) and are accompanied by quantum mechanical calculations of the predicted linear absorption spectra at the B3LYP/6-311+G(d,p) (Zn2+ complexes) and B3LYP/def2TZVP levels (Cd2+ complexes). On the basis of these experiments and calculations, the conformation that best reproduces the IRMPD spectra for the complexes of the deprotonated amino acids, [M(Cys-H)]+ and [M(CysOMe-H)]+, is a charge-solvated (CS) tridentate structure where the metal dication binds to the amine and carbonyl groups of the amino acid backbone and the deprotonated sulfur atom of the side chain, [N,CO,S−]. The intact amino acid complex, CdCl+(CysOMe) binds in the equivalent motif [N,CO,S]. These binding motifs are in agreement with the predicted ground structures of these complexes at the B3LYP, B3LYP-GD3BJ (with empirical dispersion corrections), B3P86, and MP2(full) levels.
- This article is part of the themed collection: Optical spectroscopy coupled with mass spectrometry methods

 Please wait while we load your content...
Please wait while we load your content...