Theoretical and experimental studies on the atmospheric degradation of 2-bromo-3,3,3-trifluoropropene†
Abstract
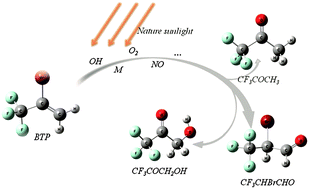
As a new kind of Halon replacement, 2-bromo-3,3,3-trifluoropropene (2-BTP) is finding application as a fire extinguishing agent in confined spaces. For assessing its environmental impact, it is necessary to perform kinetic and product studies of its degradation in the atmospheric environment. In this sense, five possible reaction pathways between 2-BTP and OH radicals are found by Gaussian 03. Detailed analysis shows that the main product is the CF3CBrCH2OH radical, which may produce a series of compounds by further reaction with O2, NO, etc. In order to further prove the validity of the theoretical calculations and investigate the atmospheric transformation process of 2-BTP, atmospheric degradation of 2-BTP is then studied experimentally under controlled radiation conditions. Based on the theoretical analyses and experimental results, the atmospheric degradation mechanism of 2-BTP is finally proposed and detailed information on the atmospheric chemistry of 2-BTP is provided.

 Please wait while we load your content...
Please wait while we load your content...