A model for ultra-fast charge transport in membrane proteins
Abstract
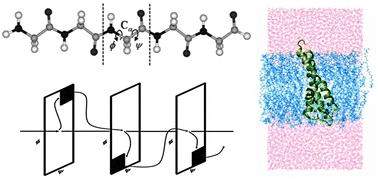
Isolated proteins have recently been observed to transport charge and reactivity over very long distances with extraordinary rates and near perfect efficiencies in spite of their site. This is not the case if the peptide is in water, where the efficiency of charge hopping to the next site is reduced to approximately 2%. Here, water is not an ideal solvent for charge transport. The issue at hand is how to explain such enormous charge transfer quenching in water compared to another typical medium, namely lipid. We performed molecular dynamics simulations to computationally substantiate the novel long-distance charge transfer yield of the polypeptides in lipids. This is characterized by the charge transfer persistent-distance decay constant and not by the rate, which is seldom, if ever, measured and hence not directly addressed here. This model can encompass an extremely wide range of yields over very long distances in peptides in various media. The calculations here demonstrate the good charge transport efficiency in lipids in contrast to the poor efficiency in water. The protein charge transport also exhibits a very strong anisotropic effect in lipids. The peptide secondary structure effect of charge transfer in membranes is analyzed in contrast to that in water. These results suggest that this model can be useful for the prediction of charge transfer efficiency in various environments of interest and indicate that the charge transfer is highly efficient in membrane proteins.

 Please wait while we load your content...
Please wait while we load your content...