The rate constant of the reaction NCN + H2 and its role in NCN and NO modeling in low pressure CH4/O2/N2-flames
Abstract
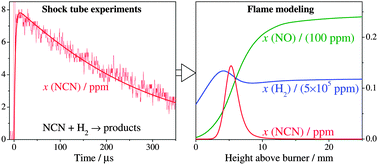
Bimolecular reactions of the NCN radical play a key role in modeling prompt-NO formation in hydrocarbon flames. The rate constant of the so-far neglected reaction NCN + H2 has been experimentally determined behind shock waves under pseudo-first order conditions with H2 as the excess component. NCN3 thermal decomposition has been used as a quantitative high temperature source of NCN radicals, which have been sensitively detected by difference UV laser absorption spectroscopy at ![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) = 30383.11 cm−1. The experiments were performed at two different total densities of ρ ≈ 4.1 × 10−6 mol cm−3 and ρ ≈ 7.4 × 10−6 mol cm−3 (corresponding to pressures between p = 324 mbar and p = 1665 mbar) and revealed a pressure independent reaction. In the temperature range 1057 K < T < 2475 K, the overall rate constant can be represented by the Arrhenius expression k/(cm3 mol−1 s−1) = 4.1 × 1013 exp(−101 kJ mol−1/RT) (Δlog k = ±0.11). The pressure independent reaction as well as the measured activation energy is consistent with a dominating H abstracting reaction channel yielding the products HNCN + H. The reaction NCN + H2 has been implemented together with a set of reactions for subsequent HNCN and HNC chemistry into the detailed GDFkin3.0_NCN mechanism for NOx flame modeling. Two fuel-rich low-pressure CH4/O2/N2-flames served as examples to quantify the impact of the additional chemical pathways. Although the overall NCN consumption by H2 remains small, significant differences have been observed for NO yields with the updated mechanism. A detailed flux analysis revealed that HNC, mainly arising from HCN/HNC isomerization, plays a decisive role and enhances NO formation through a new HNC → HNCO → NH2 → NH → NO pathway.
= 30383.11 cm−1. The experiments were performed at two different total densities of ρ ≈ 4.1 × 10−6 mol cm−3 and ρ ≈ 7.4 × 10−6 mol cm−3 (corresponding to pressures between p = 324 mbar and p = 1665 mbar) and revealed a pressure independent reaction. In the temperature range 1057 K < T < 2475 K, the overall rate constant can be represented by the Arrhenius expression k/(cm3 mol−1 s−1) = 4.1 × 1013 exp(−101 kJ mol−1/RT) (Δlog k = ±0.11). The pressure independent reaction as well as the measured activation energy is consistent with a dominating H abstracting reaction channel yielding the products HNCN + H. The reaction NCN + H2 has been implemented together with a set of reactions for subsequent HNCN and HNC chemistry into the detailed GDFkin3.0_NCN mechanism for NOx flame modeling. Two fuel-rich low-pressure CH4/O2/N2-flames served as examples to quantify the impact of the additional chemical pathways. Although the overall NCN consumption by H2 remains small, significant differences have been observed for NO yields with the updated mechanism. A detailed flux analysis revealed that HNC, mainly arising from HCN/HNC isomerization, plays a decisive role and enhances NO formation through a new HNC → HNCO → NH2 → NH → NO pathway.

 Please wait while we load your content...
Please wait while we load your content...