The electronic properties of mixed valence hydrated europium chloride thin film
Abstract
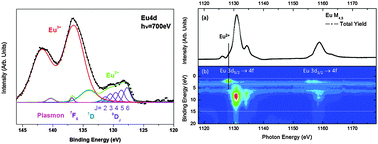
We investigate the electronic properties of a model mixed-valence hydrated chloride europium salt by means of high resolution photoemission spectroscopy (HRPES) and resonant photoemission spectroscopy (RESPES) at the Eu 3d → 4f and 4d → 4f transitions. From the HRPES spectra, we have determined that the two europium oxidation states are homogeneously distributed in the bulk and that the hydrated salt film is exempt from surface mixed valence transition. From the RESPES spectra, the well separated resonant contributions characteristic of divalent and trivalent europium species (4f6 and 4f7 final states, respectively) are accurately extracted and quantitatively determined from the resonant features measured at the two edges. The partial absorption yield spectra, obtained by integrating the photoemission intensity in the valence-band region, can be well reproduced by atomic multiplet calculation at the M4,5 (3d–4f) absorption edge and by an asymmetric Fano-like shape profile at the N4,5 (4d–4f) absorption edge. The ratio of Eu2+ and Eu3+ species measured at the two absorption edges matches with the composition of the mixed valence europium salt as determined chemically. We have demonstrated that the observed spectroscopic features of the mixed valence salt are attributed to the mixed-valence ground state rather than surface valence transition. HRPES and RESPES spectra provide reference spectra for the study of europium salts and their derivatives.

 Please wait while we load your content...
Please wait while we load your content...