The first UV absorption band for indole is not due to two simultaneous orthogonal electronic transitions differing in dipole moment
Abstract
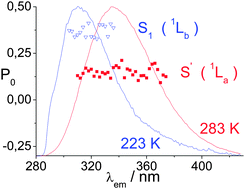
The currently accepted model for the photophysics of indole assumes that the first UV absorption band encompasses two orthogonal electronic transitions (1Lb and 1La), leading to two electronic states with a markedly different dipole moment. However, there is a body of evidence not explained by this model, which led us to develop a new photophysical model for indole. Based on the new model, the polarity of the electronic ground state (S0) in indoles is very similar to that of the first electronic excited state (S1) producing this structured emission; however, this excited state can lead to a highly dipolar excited state (S1′) with largely structureless emission under the influence of the polarity of the medium, and also, very likely, of its viscosity. The molecular structure of the new excited state can be reversibly converted into the normal structure of the compound. Previous observations were confirmed by the absorption, emission, and excitation spectra for indole, as well as by its polarized emission and excitation spectra in various media. Thus, the polarized emission spectra for indole in glycerol at 283 K and 223 K showed the transition dipole moments for the emission from the first two excited states in a polar medium, S1 and S1′, to differ by less than 20°.

 Please wait while we load your content...
Please wait while we load your content...