Fragmentation mechanism of the generation of colloidal copper(i) iodide nanoparticles by pulsed laser irradiation in liquids
Abstract
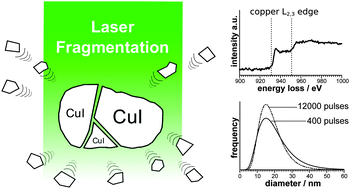
Pulsed laser ablation in liquids (PLAL) is a versatile route to stable colloids without the need for stabilizing agents. The use of suspensions instead of bulk targets further simplifies the experimental set-up and even improves the productivity. However, the utilization of this approach is hindered by limited knowledge about the underlying mechanisms of the nanoparticle formation. We present the synthesis of copper(I) iodide nanoparticles via ns-pulsed laser irradiation of CuI powder suspended in water or ethyl acetate. A thorough study of the nanoparticle size by transmission electron microscopy reveals a log-normal distribution with a mean diameter of 31 nm (±11 nm) in water and 18 nm (±7 nm) in ethyl acetate. The duration of the laser irradiation appears to have only a minor influence on the size distribution. Selected area diffraction and electron energy-loss spectroscopy verify the chemical composition of the generated CuI nanoparticles. While comparable precursors like CuO and Cu3N follow a reductive ablation mechanism, a fragmentation mechanism is found for CuI. With a productivity of 1.7 μg J−1 this pulsed laser fragmentation in liquids (PLFL) proves to be an efficient route to colloidal CuI nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...