Dissociation of CO2 on rhodium nanoclusters (Rh13) in various structures supported on unzipped graphene oxide – a DFT study
Abstract
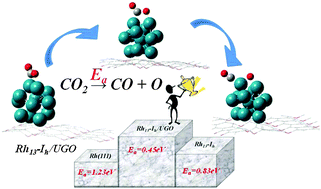
The catalytic activity of rhodium nanoclusters (Rh13) on unzipped graphene oxide (Rh13/UGO) has been investigated for comparison with Rh13 nanoclusters and Rh(111) surfaces. The binding energy of Rh atoms on UGO is less than the cohesive energy (−5.75 eV) of bulk Rh, indicating that the Rh atoms adsorbed on UGO tend to collect into clusters. We systematically calculated the adsorption energies of CO2 on Rh13 nanoclusters in various stable shapes on unzipped graphene oxide; Rh13-Ih/UGO had the highest energy (where the Ih represents icosahedral shape), −1.18 eV, with the C–O bond being elongated from 1.17 to 1.29 Å; the barrier to dissociation of CO2 on Rh13-Ih/UGO is, accordingly, the smallest (Ea = 0.45 eV), indicating that Rh13-Ih/UGO might act as an effective material to adsorb and activate the scission of the C–O bond of CO2. The calculated data required to support all evidence of this result, including the electronic distribution and the density of states, are provided.

 Please wait while we load your content...
Please wait while we load your content...