Role of the nanocrystallinity on the chemical ordering of CoxPt100−x nanocrystals synthesized by wet chemistry
Abstract
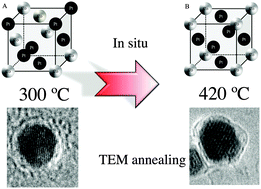
CoxPt100−x nanoalloys have been synthesized by two different chemical processes either at high or at low temperature. Their physical properties and the order/disorder phase transition induced by annealing have been investigated depending on the route of synthesis. It is demonstrated that the chemical synthesis at high temperature allows stabilization of the fcc structure of the native nanoalloys while the soft chemical approach yields mainly poly or non crystalline structure. As a result the approach of the order/disorder phase transition is strongly modified as observed by high-resolution transmission electron microscopy (HR-TEM) studies performed during in situ annealing of the different nanoalloys. The control of the nanocrystallinity leads to significant decrease in the chemical ordering temperature as the ordered structure is observed at temperatures as low as 420 °C. This in turn preserves the individual nanocrystals and prevents their coalescence usually observed during the annealing necessary for the transition to an ordered phase.
- This article is part of the themed collection: Recent advances in the chemical physics of nanoalloys

 Please wait while we load your content...
Please wait while we load your content...