Electrochemical reduction of an Ag2VO2PO4 particle: dramatic increase of local electronic conductivity
Abstract
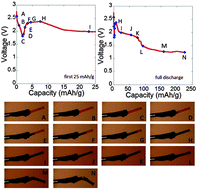
Previously, we reported that electrodes containing silver vanadium phosphate (Ag2VO2PO4) powder exhibit a 15 000 fold increase in conductivity after discharge, concurrent with the formation of silver metal. In this study, in order to disentangle the complex nature of electrodes composed of electroactive powders, an electrochemical reduction of individual particles of Ag2VO2PO4 was conducted, to more directly probe the intrinsic materials properties of Ag2VO2PO4. Specifically, individual particle conductivity data from a nanoprobe system combined with SEM and optical imaging results revealed that the depth of discharge within an Ag2VO2PO4 particle is closely linked to the conductivity increase. Notably, the formation of silver metal may affect both inter- and intraparticle conductivity of the Ag2VO2PO4 material.

 Please wait while we load your content...
Please wait while we load your content...