Collision-induced dissociation products of the protonated dipeptide carnosine: structural elucidation, fragmentation pathways and potential energy surface analysis†
Abstract
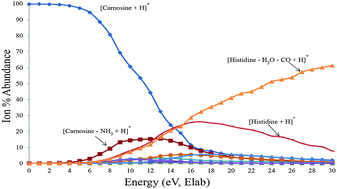
Collision-induced dissociation (CID) experiments on protonated carnosine, [carnosine + H]+, with several collision energies were shown to yield eleven different fragment ions with the generation of product ions [carnosine–H2O + H]+ and [carnosine–NH3 + H]+ being the lowest energy processes. Energy-resolved CID showed that at slightly higher collision energies the ions [histidine + H]+ and [histidine–H2O–CO + H]+ are formed. At even higher energies four other product ions were observed, however, attained relatively lower abundances. Quantum chemistry calculations, carried out at different levels of theory, were employed to probe fragmentation mechanisms that account for all the experimental data. All the adopted computational protocols give similar energetic trends, and the range of the calculated free energy barrier values for the generation of all the observed product ions is in agreement with the fragmentation mechanisms offered here.

 Please wait while we load your content...
Please wait while we load your content...