Towards a comprehensive insight into efficient hydrogen production by self-assembled Ru(bpy)32+–polymer–Pt artificial photosystems†
Abstract
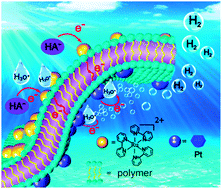
The role of polymers in artificial photosystems has been studied in detail. The photosystems were composed of tris(2,2′-bipyridyl) ruthenium(II) chloride as a photosensitizer (PS), colloidal Pt stabilized by polymer as a hydrogen-evolving catalyst and sodium ascorbate as an electron donor, without the addition of a traditional molecular electron mediator. Comprehensive insights into the production of hydrogen on irradiation with visible light were achieved. Several polymers, including neutral polyvinyl pyrrolidone, anionic poly(sodium 4-styrene sulfonate) and poly(acrylic acid) not only stabilized the nanoparticles, but were also effective in the production of hydrogen. Under the optimum conditions, an outstanding apparent quantum efficiency of 12.8% for the evolution of hydrogen was achieved. The formation of self-assembled and spatially separated donor–acceptor complexes via the non-covalent intermolecular interaction between PS and the polymer–Pt was pivotal in the efficient conversion of solar energy to hydrogen fuel. Important details of the photo-induced electron and energy transfer processes in the self-assembled artificial photosystems were determined by nanosecond transient absorption spectrometry and time-resolved fluorescence spectrometry. The initial step in the photo-catalytic production of hydrogen was a reductive quenching of the triplet excited state of the PS by sodium ascorbate, leading to a reduced form of PS, which could then be quickly quenched by the polymer. The rate-determining step was the electron transfer from PS to the catalyst via the polymer bridge.

 Please wait while we load your content...
Please wait while we load your content...