Insight into the formation mechanism of graphene quantum dots and the size effect on their electrochemical behaviors†
Abstract
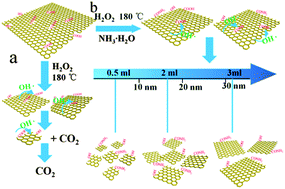
To study the formation mechanism and influencing factors of graphene quantum dots (GQDs), GQDs with different average sizes were prepared using a modified hydrothermal method with hydrogen peroxide (H2O2) as an etching agent and ammonia as an assistant. It is found that size-controlled GQDs were prepared by adjusting the amount of ammonia and porous reduced graphene oxide (PRGO) debris can be synthesized by reducing the hydrothermal reaction time. Structural changes of final products were mainly attributed to the changes in the etching ability of the hydroxyl radical (OH˙) against the reduction ability of the hydroxyl group (OH−) in different alkaline environments regulated by ammonia. Furthermore, we studied the electrochemical properties of GQDs and PRGO. The results showed that the specific capacitance of all samples increases linearly with the size and the smallest GQDs can work at the highest scan rate of as high as 5000 V s−1 with an ultra-fast power response (τ0 = 63.3 μs). Thus, these findings elucidate the formation mechanism of GQDs and demonstrate that GQDs are applicable in microelectronic devices with high power response requirements.

 Please wait while we load your content...
Please wait while we load your content...