Equilibration processes during gas uptake inside narrow pores
Abstract
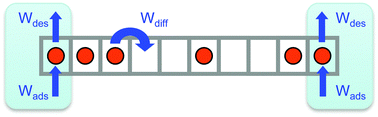
We analyze the adsorption kinetics of a gas in contact with the open ends of a narrow longitudinal pore, where gas transport along its interior occurs via single-file diffusion mechanisms. By implementing a Kinetic Monte Carlo simulation of the gas dynamics, we obtain the overall change in gas uptake inside the pore and the concentration profile of the adsorbed phase as the system evolves towards equilibrium. Typically, higher external pressure leads to faster kinetics as it happens for adsorption on open surfaces. However, when the pore is exposed to gas at very high pressures, blockage events near the ends of longer pores can slow down the overall adsorption, with desorption and internal diffusion eventually becoming the rate limiting processes. We determine the dependence of these phenomena on the amount of gas adsorbed, binding energy and length of the pore.

 Please wait while we load your content...
Please wait while we load your content...