A QCM study of ORR-OER and an in situ study of a redox mediator in DMSO for Li–O2 batteries†
Abstract
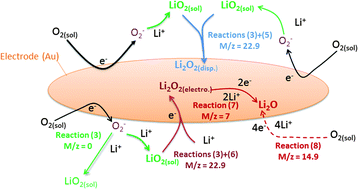
The oxygen reduction reaction and oxygen evolution reaction (ORR-OER) in DMSO were investigated by cyclic voltammetry and potentiostatic methods. A quartz crystal microbalance (QCM) was used to detect which products are formed during reduction and to evaluate the reversibility of the reactions. The studied parameters include the scan rate and the applied cathodic potential. We confirm by the QCM that LiO2 is soluble: this conclusion comes from the time delay we observed between the deposition of the expected mass (based on Faraday's law) and the measured mass. Ambiguity in reported literature values for the slope of the deposited mass per electron 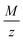 is due to the negligence in considering this time delay. The average
is due to the negligence in considering this time delay. The average 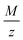 value versus cathodic charge indicates that soluble LiO2 is the first product of the ORR which reacts further to form Li2O2, either via a disproportionation reaction or via further electrochemical reduction of LiO2. For strong negative potentials and thus large depths of discharge, Li2O is the main discharge product. The reaction pathways hence strongly depend on the experimental conditions applied; especially the reduction potential. The redox mediator tetrathiafulvalene (TTF) was investigated and its influence on reversibility was confirmed by cycling at moderate depths of discharge, where Li2O2 is the main discharge product.
value versus cathodic charge indicates that soluble LiO2 is the first product of the ORR which reacts further to form Li2O2, either via a disproportionation reaction or via further electrochemical reduction of LiO2. For strong negative potentials and thus large depths of discharge, Li2O is the main discharge product. The reaction pathways hence strongly depend on the experimental conditions applied; especially the reduction potential. The redox mediator tetrathiafulvalene (TTF) was investigated and its influence on reversibility was confirmed by cycling at moderate depths of discharge, where Li2O2 is the main discharge product.

 Please wait while we load your content...
Please wait while we load your content...