Strong electronic polarization of the C60 fullerene by imidazolium-based ionic liquids: accurate insights from Born–Oppenheimer molecular dynamic simulations
Abstract
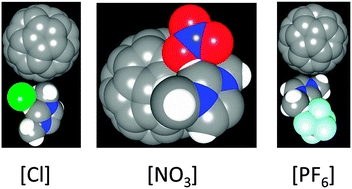
Fullerenes are known to be polarizable due to their strained carbon–carbon bonds and high surface curvature. The electronic polarization of fullerenes is steadily of practical importance because it leads to non-additive interactions and, therefore, to unexpected phenomena. For the first time, hybrid density functional theory (HDFT) powered Born–Oppenheimer molecular dynamics (BOMD) simulations have been conducted to observe electronic polarization and charge transfer phenomena in the C60 fullerene at finite temperature (350 K). The non-additive phenomena are fostered by the three selected imidazolium-based room-temperature ionic liquids (RTILs). We conclude that although charge transfer appears nearly negligible in these systems, electronic polarization is indeed significant, leading to a systematically positive effective electrostatic charge on the C60 fullerene: +0.14e in [MMIM][Cl], +0.21e in [MMIM][NO3], and +0.17e in [MMIM][PF6]. These results are, to a certain extent, unexpected and provide a motivation for considering novel C60–RTILs systems. HDFT BOMD is a powerful tool for investigating electronic effects in RTIL and fullerene containing nuclear-electronic systems.

 Please wait while we load your content...
Please wait while we load your content...