Anion and cation dynamics of sulfonylamide-based ionic liquids and the solid–liquid transitions
Abstract
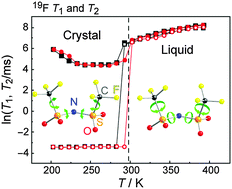
Some of the important factors that characterise room-temperature ionic liquids (RTILs) are the variety of conformations adopted by the constituent ions and their flexibility. Using 1,3-dimethylimidazolium bis(fluorosulfonyl)amide ([C1mim][FSA]) and 1,3-dimethylimidazolium bis(trifluoromethylsulfonyl)amide ([C1mim][NTf2]) as samples, the longitudinal and transverse relaxation times (T1 and T2) for 19F and 1H were determined as a function of temperature and were correlated with the dynamics of the phase behaviours of the two RTILs. Because the anions and cations in the two compounds have 19F and 1H nuclei, respectively, their dynamics can be independently investigated and the relationships between them can be discussed. For [C1mim][FSA], the only observed phase changes included melting and crystallisation. The temperature dependences of T1 and T2 for 19F were similar to those of T1 and T2 for 1H, indicating similar dynamics due to the formation of strong anion–cation interactions. For [C1mim][NTf2], the T1 and T2 values for both 19F and 1H discontinuously changed at same temperatures, which were assigned to the crystallisation and melting points. However, the T1 curves for 19F and 1H were different in the crystalline region, suggesting independent dynamics for the anions and cations in [C1mim][NTf2]. In the crystalline state for each salt, the cation dynamics was distinctly separated into the framework movement of the imidazolium ring and the movement of the methyl groups, while the anion dynamics was characterised by the movement of the entire anion. The influence of the crystal structure on the dynamics of each salt was also considered.

 Please wait while we load your content...
Please wait while we load your content...