Interfacial water at the trialanine hydrophilic surface: a DFT electronic structure and bottom-up investigation†‡
Abstract
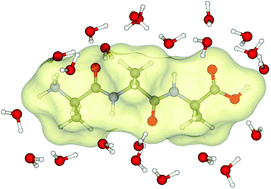
DFT-M062X quantum chemical computations on the Ala3H+·nH2O (n up to 37) complexes have been performed to model for hydration effects on the molecular properties of protonated trialanine. Following simple rules to arrange water molecules around the peptide, geometry optimization allows us to find four minima corresponding to the unfolded extended (β) and polyproline II (PPII) conformations. The peptide is incorporated into the network of hydrogen bonds of interfacial water molecules with a hydration energy of about −85 kcal mol−1. The progressive hydration of the peptide shows a more efficient intermolecular hydrogen bonding in the PPII arrangement, and the following relative electronic energy stability β–β < β-PPII ≈ PPII-β < PPII–PPII has been found. The conformational entropy term proceeds in the reverse direction, thus these changes compensate in a way that leads to small changes in Gibbs free energy. These findings agree with experimental data which report an equilibrium between these conformers modulated by temperature.


 Please wait while we load your content...
Please wait while we load your content...