Mechanism of visible light photocatalytic NOx oxidation with plasmonic Bi cocatalyst-enhanced (BiO)2CO3 hierarchical microspheres†
Abstract
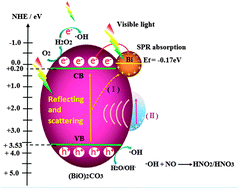
Semimetal bismuth (Bi), as an emerging non-noble metal-based cocatalyst and plasmonic photocatalyst, has attracted significant attention. In this work, a one-pot solvent-controlled synthesis strategy was utilized for the in situ-deposition of plasmonic Bi nanoparticles onto the surfaces of (BiO)2CO3 microspheres (BOC-WE) using bismuth citrate, sodium carbonate, and ethylene glycol as precursors. The introduction of the Bi nanoparticles has a pivotal effect on the morphology, optical and photocatalytic performance of the pristine (BiO)2CO3. The results indicated that the Bi nanoparticles were generated on the surface of (BiO)2CO3 microspheres via the in situ reduction of Bi3+ by ethylene glycol. The Bi-deposited (BiO)2CO3 microspheres were used for the photocatalytic purification of NOx in air under visible light irradiation. Significantly, the BOC-WE samples exhibited a drastically promoted photocatalytic performance with a NOx removal ratio (η) of 37.2%, superior to pristine (BiO)2CO3 (η = 19.1%), outperforming other well-known visible light photocatalysts, such as C-doped TiO2 (η = 21.8%), BiOBr (η = 21.3%), BiOI (η = 14.9%) and C3N4 (η = 25.5%). The conspicuously enhanced photocatalytic capability can be attributed to the synergistic effects of the surface plasmon resonance (SPR) effect, increased visible light absorption and the efficient separation of electron–hole pairs induced by the Bi nanoparticles. The Bi nanoparticles can act as a non-noble metal-based cocatalyst for strengthening photocatalytic performance, which is similar to the behavior of noble metals (Au, Ag) in enhancing photocatalysis. The mechanism of visible light photocatalytic NOx oxidation was investigated. DMPO-ESR spin-trapping results demonstrated that hydroxyl radicals were confirmed to be the main active species for NOx photo-oxidation. Due to the SPR effect of Bi, the BOC-WE could produce more hydroxyl radicals than BOC, which was responsible for the enhanced NO photo-oxidation ability. Moreover, the BOC-WE photocatalysts showed high photochemical stability under repeated irradiation. This work demonstrates the feasibility of utilizing low cost Bi cocatalysts as a substitute for noble metals to enhance the performance of other photocatalysts. This work could not only provide new insights into the in situ fabrication of Bi/semiconductor nanocomposites, but also pave a new way for the modification of photocatalysts with non-noble metals as cocatalysts to achieve an enhanced performance for environmental and energetic applications.

 Please wait while we load your content...
Please wait while we load your content...