Structural properties of methanol–water binary mixtures within the quantum cluster equilibrium model†
Abstract
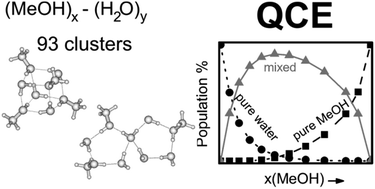
Density functional theory (B3LYP-D3, M06-2X) has been used to calculate the structures, interaction energies and vibrational frequencies of a set of 93 methanol–water clusters of different type (cubic, ring, spiro, lasso, bicyclic), size and composition. These interaction energies have been used within the framework of the Quantum Cluster Equilibrium Theory (QCE) to calculate cluster populations as well as thermodynamic properties of binary methanol–water mixtures spanning the whole range from pure water to pure methanol. The necessary parameters amf and bxv of the QCE model were obtained by fitting to experimental isobars of MeOH–H2O mixtures with different MeOH content. The cubic and spiro motifs dominate the distribution of methanol–water clusters in the mixtures with a maximum of mixed clusters at x(MeOH) = 0.365. Reasonable agreement with experimental data as well as earlier molecular dynamics simulations was found for excess enthalpies HE, entropies SE as well as Gibbs free energies of mixing GE. In contrast, heat capacities Cp and CEp showed only poor agreement with experimental data.
- This article is part of the themed collection: Bunsentagung 2015: Solvation Science

 Please wait while we load your content...
Please wait while we load your content...