A sodium ion intercalation material: a comparative study of amorphous and crystalline FePO4†
Abstract
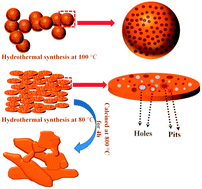
Due to their low cost, high abundance and eco-friendly features, Na-ion batteries are becoming alternative choices for rechargeable batteries, especially in large scale applications. Generally, the well-crystallized materials have many advantages over amorphous materials, such as long cycle life, high rate performance and other electrochemical properties. However, the amorphous FePO4 we report here exhibits outstanding cycling stability and rate performance which are derived from its amorphous nature and wafer-like porous morphology. A comparative study of amorphous and crystalline FePO4 has been carried out as cathode materials for Na-ion batteries. The present study not only reports a synthetic method which is facile, inexpensive, and scalable for mass production, but it also motivates further exploration of other amorphous materials for Na-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...