Collisional relaxation of apocarotenals: identifying the S* state with vibrationally excited molecules in the ground electronic state S0*†
Abstract
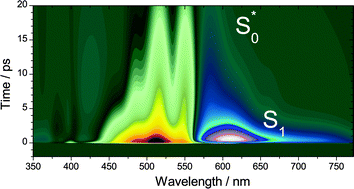
In recent work, we demonstrated that the S* signal of β-carotene observed in transient pump–supercontinuum probe absorption experiments agrees well with the independently measured steady-state difference absorption spectrum of vibrationally hot ground state molecules S0* in solution, recorded at elevated temperatures (Oum et al., Phys. Chem. Chem. Phys., 2010, 12, 8832). Here, we extend our support for this “vibrationally hot ground state model” of S* by experiments for the three terminally aldehyde-substituted carotenes β-apo-12′-carotenal, β-apo-4′-carotenal and 3′,4′-didehydro-β,ψ-caroten-16′-al (“torularhodinaldehyde”) which were investigated by ultrafast pump–supercontinuum probe spectroscopy in the range 350–770 nm. The apocarotenals feature an increasing conjugation length, resulting in a systematically shorter S1 lifetime of 192, 4.9 and 1.2 ps, respectively, in the solvent n-hexane. Consequently, for torularhodinaldehyde a large population of highly vibrationally excited molecules in the ground electronic state is quickly generated by internal conversion (IC) from S1 already within the first picosecond of relaxation. As a result, a clear S* signal is visible which exhibits the same spectral characteristics as in the aforementioned study of β-carotene: a pronounced S0 → S2 red-edge absorption and a “finger-type” structure in the S0 → S2 bleach region. The cooling process is described in a simplified way by assuming an initially formed vibrationally very hot species S0** which subsequently decays with a time constant of 3.4 ps to form a still hot S0* species which relaxes with a time constant of 10.5 ps to form S0 molecules at 298 K. β-Apo-4′-carotenal behaves in a quite similar way. Here, a single vibrationally hot S0* species is sufficient in the kinetic modeling procedure. S0* relaxes with a time constant of 12.1 ps to form cold S0. Finally, no S0* features are visible for β-apo-12′-carotenal. In that case, the S1 → S0 IC process is expected to be roughly 20 times slower than S0* relaxation. As a result, no spectral features of S0* can be found, because there is no chance that a detectable concentration of vibrationally hot molecules is accumulated.

 Please wait while we load your content...
Please wait while we load your content...