Excited state evolution of DNA stacked adenines resolved at the CASPT2//CASSCF/Amber level: from the bright to the excimer state and back†
Abstract
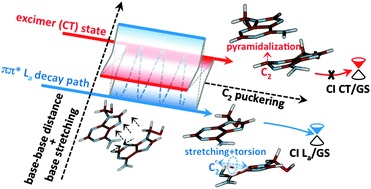
Deactivation routes of bright ππ* (La) and excimer charge transfer (CT) states have been mapped for two stacked quantum mechanical (CASPT2//CASSCF) adenines inside a solvated DNA double strand decamer (poly(dA)·poly(dT)) described at the molecular mechanics level. Calculations show that one carbon (C2) puckering is a common relaxation coordinate for both the La and CT paths. By mapping the lowest crossing regions between La and CT states, together with the paths connecting the two states, we conclude that at least one CT state can be easily accessible. The lowest-lying conical intersections between ground state (GS) and CT states have been fully characterized in a realistic DNA environment for the first time. We show that the path to reach this crossing region from the CT minima involves high barriers that are not consistent with experimental data lifetimes. Instead, the multiexponential decay recorded in DNA, including the longest (ca. 100 picoseconds) lifetime component detected in oligomeric single- and double-stranded systems, is compatible with both intra-monomer relaxation processes along the La deactivation path (involving small barriers) and the population of the excimer (CT) state that behaves as a trap. In the latter case, deactivation is feasible only going back to the La state by following its preferred decay coordinate.

 Please wait while we load your content...
Please wait while we load your content...