Theoretical study on the torsional potential of alkyl, donor, and acceptor substituted bithiophene: the hidden role of noncovalent interaction and backbone conjugation†
Abstract
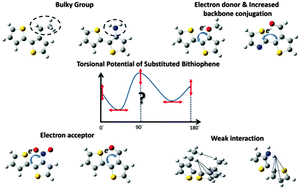
Side chain and substituent engineering of conjugated polymers are important to their backbone design. Of particular interest here is how side chains and substituents influence the coplanarity of conjugated backbones. Steric hindrance is usually considered as the principal factor influencing the coplanarity. In this study, we used proper first-principle density functional theories to analyze the change in the torsional potentials of substituted bithiophene with substituents of varying degrees of electron donating/accepting capabilities. Besides steric hindrance, the torsional potential of substituted bithiophene is also determined by other factors such as the position of substitution, non-covalent interactions between the substituents and thiophene ring, and electron conjugation in the backbone. There is no significant change in the torsional potential unless the substituent group is located at the head position of bithiophene. The bulkiness of the substituent group increases the torsional barrier at 0 and 180 degree (planar bithiophene), while the weak noncovalent interaction (such as CH–π, NH–π, and dispersion interactions) stabilizes the transition structure and decreases the barrier at 90 degree (two thiophene rings in perpendicular). Strong electron-withdrawing substituent groups (e.g., formyl or nitro groups) are found to reduce backbone conjugation resulting in reduced internal rotation barrier at 90 degree. Any of these factors deteriorates the coplanarity of bithiophene. On the other hand, the backbone conjugation can be enhanced by introducing electron-donating groups (e.g., methoxy) resulting in an increased internal rotational barrier and stabilized planar structure. The influence of through-space interactions such as S⋯O, S⋯N and CH⋯O interactions are found to play a minor role in the coplanarity of substituted bithiophene.

 Please wait while we load your content...
Please wait while we load your content...