Solution vs. gas phase relative stability of the choline/acetylcholine cavitand complexes†
Abstract
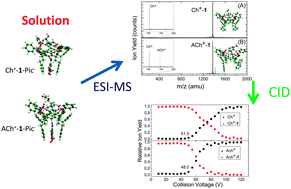
How the information obtained from the gas phase experiments can reflect the processes in solution is a crucial question for analytical chemistry, and particularly the selective host–guest recognition mechanisms which are fundamental in biology. Here we combine ElectroSpray Ionization mass spectrometry (ESI-MS) and the Collision Induced Dissociation (CID) experiments to the density functional theory to investigate the interaction of acetylcholine and the choline cation with a triphosphonate cavitand. While the relative abundance of the cation complexes in the ESI mass spectrum reflects the preferential capture of the acetylcholine ion over the choline ion by the cavitand in the solution, the gas phase CID measurements indicate that after desolvation the choline cation is the most strongly bound to the host. The experimental results are interpreted by theory that underlines the role of the counterion in the stabilization of the complexes in solution and therefore in the selective recognition of substrates of biological interest.

 Please wait while we load your content...
Please wait while we load your content...