Novel 2-alkyl-1-ethylpyridinium ionic liquids: synthesis, dissociation energies and volatility†
Abstract
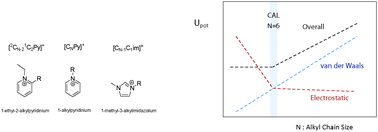
This work presents the synthesis, volatility study and electrospray ionization mass spectrometry with energy-variable collision induced dissociation of the isolated [(cation)2(anion)]+ of a novel series of 2-alkyl-1-ethyl pyridinium based ionic liquids, [2CN−21C2Py][NTf2]. Compared to the imidazolium based ionic liquids, the new ionic liquid series presents a higher thermal stability and lower volatility. The [(cation)2(anion)]+ collision induced dissociation energies of both [2CN−21C2Py][NTf2] and [CNPy][NTf2] pyridinium series show an identical trend with a pronounced decrease of the relative cation–anion interaction energy towards an almost constant value for N = 6. It was found that the lower volatility of [2CN−21C2Py][NTf2] with a shorter alkyl chain length is due to its higher enthalpy of vaporization. Starting from [2C31C2Py][NTf2], the lower volatility is governed by the combination of slightly lower entropies and higher enthalpies of vaporization, an indication of a higher structural disorder of the pyridinium based ionic liquids than the imidazolium based ionic liquids. Dissociation energies and volatility trends support the cohesive energy interpretation model based on the overlapping of the electrostatic and van der Waals functional interaction potentials.

 Please wait while we load your content...
Please wait while we load your content...