Chromium deposition and poisoning at La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen electrodes of solid oxide electrolysis cells
Abstract
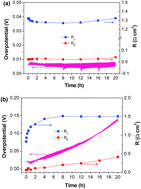
The degradation of solid oxide electrolysis cells (SOECs) is an issue of both scientific and technical importance. In this study, chromium deposition and poisoning at the La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) anode or the oxygen electrode of SOECs are studied for the first time under a current density of 200 mA cm−2 at 900 and 800 °C. After polarization in the presence of the Fe–Cr interconnect at 900 °C for 20 h, electrode polarization resistance and overpotential of the O2 evolution reaction (OER) on the LSCF electrode are 0.413 Ω cm−2 and 127 mV, respectively, which is nearly 7 and 18 times the initial values of the electrode before the polarization. Significant performance degradation was also observed for the reaction at 800 °C in the presence of the Fe–Cr alloy. XRD and XPS analyses clearly identified the deposition of SrCrO4, CrO2.5 and Cr2O3 phases on the surface of LSCF oxygen electrodes and their formation is closely related to the increased segregation of the SrO species under anodic polarization conditions. Sr segregation leads to Sr deficiency at the A-site, thus deteriorating the electrocatalytic activity of the LSCF oxygen electrodes for OER. The results indicate that Cr deposition is essentially a chemical reaction and is initiated by the nucleation reaction between the gaseous Cr species and segregated SrO on the surface region of the LSCF oxygen electrode.

 Please wait while we load your content...
Please wait while we load your content...