Why genetic modification of lignin leads to low-recalcitrance biomass†
Abstract
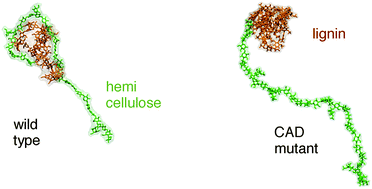
Genetic modification of plants via down-regulation of cinnamyl alcohol dehydrogenase leads to incorporation of aldehyde groups in the lignin polymer. The resulting lignocellulosic biomass has increased bioethanol yield. However, a molecular-scale explanation of this finding is currently lacking. Here, we perform molecular dynamics simulation of the copolymer with hemicellulose of wild type and the genetically modified lignin, in aqueous solution. We find that the non-covalent association with hemicellulose of lignin containing aldehyde groups is reduced compared to the wild-type. This phase separation may increase the cell wall porosity in the mutant plants, thus explaining their easier deconstruction to biofuels. The thermodynamic origin of the reduced lignin-hemicellulose association is found to be a more favorable self-interaction energy and less favorable interaction with hemicellulose for the mutant lignin. Furthermore, reduced hydration water density fluctuations are found for the mutant lignin, implying a more hydrophobic lignin surface. The results provide a detailed description of how aldehyde incorporation makes lignin more hydrophobic and reduces its association with hemicellulose, thus suggesting that increased lignin hydrophobicity may be an optimal characteristic required for improved biofuel production.

 Please wait while we load your content...
Please wait while we load your content...