Thermostability and reversibility of silver nanoparticle–protein binding†
Abstract
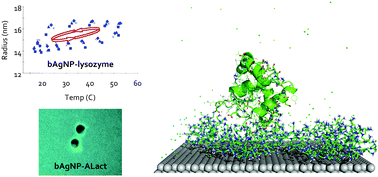
The interactions between nanoparticles (NPs) and proteins in living systems are a precursor to the formation of a NP–protein “corona” that underlies cellular and organism responses to nanomaterials. However, the thermodynamic properties and reversibility of NP–protein interactions have rarely been examined. Using an automated, high-throughput and temperature-controlled dynamic light scattering (DLS) technique we observed a distinct hysteresis in the hydrodynamic radius of branched polyethyleneimine (BPEI) coated-silver nanoparticles (bAgNPs) exposed to like-charged lysozyme during the processes of heating and cooling, in contrast to the irreversible interactions between bAgNPs and oppositely charged alpha lactalbumin (ALact). Our discrete molecular dynamics (DMD) simulations offered a new molecular insight into the differential structure, dynamics and thermodynamics of bAgNPs binding with the two protein homologs and further revealed the different roles of the capping agents of citrate and BPEI in NP–protein interactions. This study facilitates our understanding of the transformation of nanomaterials in living systems, whose implications range from the field study of nanotoxicology to nanomaterials synthesis, nanobiotechnology and nanomedicine.

 Please wait while we load your content...
Please wait while we load your content...