Nature of aryl–tyrosine interactions contribute to β-hairpin scaffold stability: NMR evidence for alternate ring geometry†
Abstract
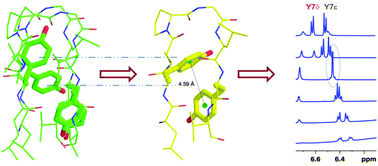
The specific contribution of the acidic-aromatic β-sheet favouring amino acid tyrosine to the stability of short octapeptide β-hairpin structures is presented here. Solution NMR analysis in near-apolar environments suggests the energetically favourable mode of interaction to be T-shaped face-to-edge (FtE) and that a Trp–Tyr interacting pair is the most stabilizing. Alternate aryl geometries also exist in solution, which readily equilibrate between a preferred π⋯π conformation to an aromatic–amide conformation, without any change in the backbone structure. While the phenolic ring is readily accommodated at the “edge” of FtE aryl interactions, it exhibits an overall lowered contribution to scaffold stability in the “face” orientation. Such differential tyrosine interactions are key to its dual nature in proteins.


 Please wait while we load your content...
Please wait while we load your content...