Electrochemical lithiation performance and characterization of silicon–graphite composites with lithium, sodium, potassium, and ammonium polyacrylate binders†
Abstract
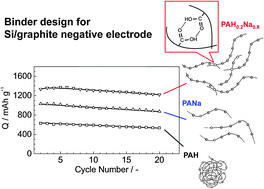
Poly(acrylic acid) (PAH), which is a water soluble polycarboxylic acid, is neutralized by adding different amounts of LiOH, NaOH, KOH, and ammonia (NH4OH) aqueous solutions to fix neutralization degrees. The differently neutralized polyacid, alkali and ammonium polyacrylates are examined as polymeric binders for the preparation of Si–graphite composite electrodes as negative electrodes for Li-ion batteries. The electrode performance of the Si–graphite composite depends on the alkali chemicals and neutralization degree. It is found that 80% NaOH-neutralized polyacrylate binder (a pH value of the resultant aqueous solution is ca. 6.7) is the most efficient binder to enhance the electrochemical lithiation and de-lithiation performance of the Si–graphite composite electrode compared to that of conventional PVdF and the other binders used in this study. The optimum polyacrylate binder highly improves the dispersion of active material in the composite electrode. The binder also provides the strong adhesion, suitable porosity, and hardness for the composite electrode with 10% (m/m) binder content, resulting in better electrochemical reversibility. From these results, the factors of alkali-neutralized polyacrylate binders affecting the electrode performance of Si–graphite composite electrodes are discussed.

 Please wait while we load your content...
Please wait while we load your content...