The properties of Irn (n = 2–10) clusters and their nucleation on γ-Al2O3 and MgO surfaces: from ab initio studies
Abstract
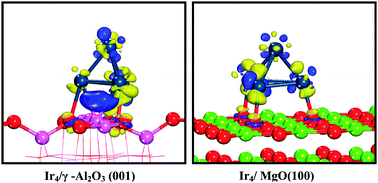
The structural stability and magnetic properties of iridium clusters Irn (n = 2–10) and their interaction on γ-Al2O3(001) and MgO(100) surfaces have been investigated from first principles calculations. It is found that the adsorption energy of Irn (n = 2–10)/γ-Al2O3(001) is lower than that of Irn/MgO(100); meanwhile, the strongest adsorption energy cluster for γ-Al2O3(001) is the tetrahedral Ir4 cluster, while for MgO(100) is a cubic Ir8 cluster. On the other hand, the nucleation of Irn (n = 2–10) clusters on both surfaces is thermodynamically favorable and exothermic. Moreover, the nucleation energy of Irn (n = 2–10) clusters on the γ-Al2O3(001) surface is close to the corresponding energy on the MgO(100) surface, except for Ir3, Ir4 and Ir6 clusters. Interestingly, the nucleation of Ir3 and Ir6 clusters on the MgO(100) surface is more favorable than that on the γ-Al2O3(001) surface, while the nucleation of the Ir4 cluster is reverse and very close to the gas phase Ir4 cluster. More importantly, deformation energy and charge density analysis show that the adsorbed Ir4 cluster on the γ-Al2O3(001) surface has obviously charge transfer between Ir atoms and surface Al, O atoms with negligible deformation. However, for the MgO(100) surface, the Ir4 cluster connects directly to three surface O atoms with severe distortion, which inhibits the activity of the tetrahedral Ir4 cluster as a catalyst.

 Please wait while we load your content...
Please wait while we load your content...