Computational investigations of the thermodynamic properties of size-selected water and Ar–water clusters: high-pressure transitions
Abstract
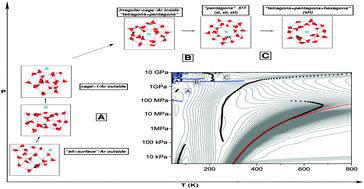
Classical parallel-tempering Monte Carlo simulations in the isothermal–isobaric ensemble were carried out for the (H2O)20 and Ar(H2O)20 clusters, over a wide range of temperatures (30–1000 K) and pressures (3 kPa–10 GPa) in order to study their thermodynamic properties and structural changes. The TIP4P/ice water model is employed for the water–water interactions, while both semiempirical and ab initio-based potentials are used to model the interaction between the rare-gas atoms and the water molecules. Temperature–pressure phase diagrams for these cluster systems were constructed by employing a two-dimensional multiple-histogram method. Structural changes were detected by analyzing the heat capacity landscape and the Pearson correlation coefficient profile for the interaction energy and volume. Those at high pressure correspond to solid-to-solid transitions and are found to be related to clathrate-like cages around the Ar atom. It is also shown that the formation and thermodynamic stability of such structures are determined by the intermolecular interaction between the rare-gas atoms and the host water molecules.

 Please wait while we load your content...
Please wait while we load your content...