Self-assembly polymorphism of 2,7-bis-nonyloxy-9-fluorenone: solvent induced the diversity of intermolecular dipole–dipole interactions†
Abstract
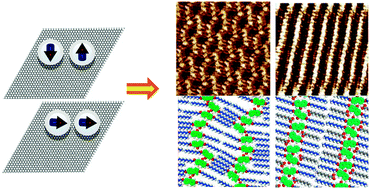
In this present work, a scanning tunneling microscope (STM) operated under ambient conditions was utilized to probe the self-assembly behavior of 2,7-bis-nonyloxy-9-fluorenone (F–OC9) at the liquid–solid (l/s) interface. On the highly oriented pyrolytic graphite (HOPG) surface, two-dimensional (2D) polymorphism with diversity of intermolecular dipole interactions induced by solvent was found. Solvents ranged from hydrophilic solvating properties with high polarity, such as viscous alkylated acids, to nonpolar alkylated aromatics and alkanes. 1-Octanol and dichloromethane were used to detect the assembly of F–OC9 at the gas–solid (g/s) interface. The opto-electronic properties of F–OC9 were determined by UV-vis and fluorescence spectroscopy in solution. Our results showed that there were tremendous solvent-dependent self-assemblies in 2D ordering for the surface-confined target molecules. When a homologous series of alkanoic acids ranging from heptanoic to nonanoic acid were employed as solvents, the self-assembled monolayer evolved from low-density coadsorbed linear lamellae to a semi-circle-like pattern at relatively high concentrations, which was proven to be the thermodynamic state as it was the sole phase observed at the g/s interface after the evaporation of solvent. Moreover, by increasing the chain length of the alkylated acids, the weight of the carboxylic group, also being the group responsible for the dielectric properties, diminished from heptanoic to nonanoic acid, which could make the easier/earlier appearance of a linear coadsorption effect. However, this was not the case for nonpolar 1-phenyloctane and n-tetradecane: no concentration effect was detected. It showed a strong tendency to aggregate to generate coexistence of separate domains of different phases due to the fast nucleation sites. Furthermore, thermodynamic calculations indicated that the stable structural coexistence of the fluorenone derivative was attributed to synergistic intermolecular dipole–dipole and van der Waals (vdWs) forces at l/s interface. It is believed that the results are of significance to the fields of solvent induced polymorphism assembly and surface science.

 Please wait while we load your content...
Please wait while we load your content...