Distinct and dramatic water dissociation on GaP(111) tracked by near-ambient pressure X-ray photoelectron spectroscopy†
Abstract
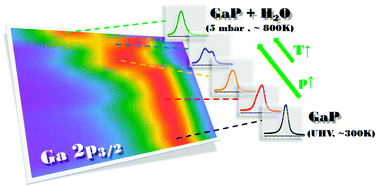
Water adsorption and dissociation on a GaP(111) crystal surface are investigated using near-ambient pressure X-ray photoelectron spectroscopy (NAP XPS) in a wide range of pressures (∼10−10–5 mbar) and temperatures (∼300–773 K). Dynamic changes in chemical evolution at the H2O/GaP(111) interface are reflected in Ga 2p3/2, O 1s, and P 2p spectra. In the pressure-dependent study performed at room temperature, an enhancement of surface Ga hydroxylation and oxidation with an increase in H2O pressure is observed. In the temperature-dependent study performed at elevated pressures, two distinct regions can be defined in which drastic changes occur in the surface chemistry. Below 673 K, the surface Ga hydroxylation and oxidation progress continuously. However, above 673 K, a large-scale conversion of surface O–Ga–OH species into non-stoichiometric Ga hydroxide along with oxidation of surface P atoms occurs through an intermediate state. The NAP XPS technique enabled us to experimentally track the chemistry at the H2O/GaP interface under near-realistic conditions, thereby providing evidence to compare with recent theoretical efforts to improve the understanding of water-splitting mechanisms and photo-corrosion on semiconductor surfaces.

 Please wait while we load your content...
Please wait while we load your content...