Microsolvation of 2-azetidinone: a model for the peptide group–water interactions†
Abstract
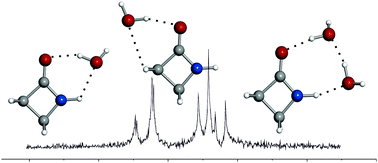
2-Azetidinone–water clusters can be considered as appropriate models for investigating the interaction of the peptide functional group with water. The rotational spectra of 2-azetidinone–(H2O)n (n = 1, 2) complexes have been studied in the 6–18 GHz frequency range using a molecular beam Fourier transform microwave spectrometer. Two different isomers have been observed for the 1 : 1 adduct. The most stable (1 : 1a) is stabilized by two hydrogen bonds O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H⋯O with water closing a ring with the peptide group. For the other conformer (1 : 1b), water is placed on the other side of the carbonyl group stabilized by O–H⋯O
C and N–H⋯O with water closing a ring with the peptide group. For the other conformer (1 : 1b), water is placed on the other side of the carbonyl group stabilized by O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H⋯O hydrogen bonds. In 2-azetidinone–(H2O)2 the water molecules close a ring with the peptide group forming three different hydrogen bonds: O–H⋯O
C and C–H⋯O hydrogen bonds. In 2-azetidinone–(H2O)2 the water molecules close a ring with the peptide group forming three different hydrogen bonds: O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, O–H⋯O and N–H⋯O. The spectra of the parent and several isotopologues of each cluster have been investigated in order to determine their structures. The hydrogen bond geometries show that the dominant interaction is the O–H⋯O
C, O–H⋯O and N–H⋯O. The spectra of the parent and several isotopologues of each cluster have been investigated in order to determine their structures. The hydrogen bond geometries show that the dominant interaction is the O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond, in good agreement with the observed preference of water to interact with this group in proteins. A comparison between the geometries of the different observed adducts shows clearly how cooperative hydrogen bonding plays an important role in the stabilization of these complexes. No detectable structural changes have been observed for 2-azetidinone upon hydration with one water molecule.
C hydrogen bond, in good agreement with the observed preference of water to interact with this group in proteins. A comparison between the geometries of the different observed adducts shows clearly how cooperative hydrogen bonding plays an important role in the stabilization of these complexes. No detectable structural changes have been observed for 2-azetidinone upon hydration with one water molecule.

 Please wait while we load your content...
Please wait while we load your content...